MoMed Biotech Announces First Batch of Activity and Animal Experiment Data for Breast Cancer PROTAC Pipeline.
Recently, MoMed Biotech's R&D team announced the first batch of activity and animal experiment data for the breast cancer PROTAC pipeline. The initial results obtained from the research, which was designed using MoMed Biotech's MechGen multidimensional intelligent technology platform, indicate that the PROTAC protein degrader molecule CZ-501, which targets ER degradation, performs excellently in terms of both efficacy and safety, with its activity and other indicators reaching the best level in the industry.
Experimental Results:
(1) Efficacy Data
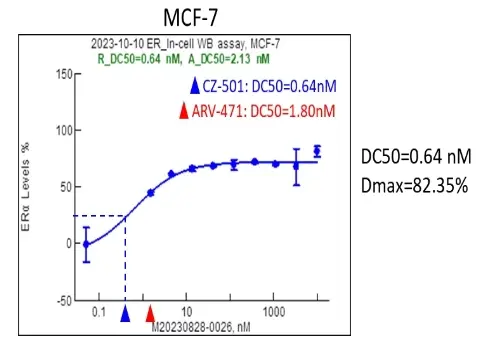
The experimental data showed that compared to ARV-471, a clinical phase III drug developed by Arvinas in the same field, CZ-501 had a higher target degradation efficiency in MCF-7 cancer cells, reaching a world-leading level. The following figure shows a comparison of the target degradation efficiency of the two PROTAC protein degraders.
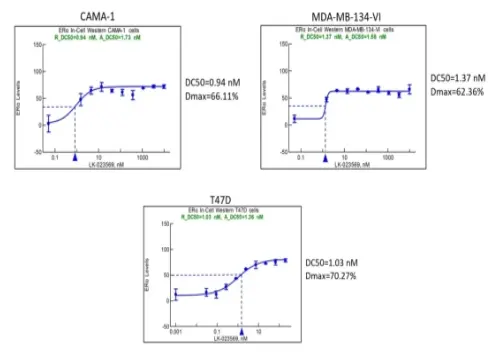
The research also indicates that besides the MCF-7 cancer cell line, CZ-501 can achieve target protein degradation in various other breast cancer cell lines (CAMA-1, MDA-MB-134-VI, T47D), demonstrating its excellent efficacy and broad-spectrum characteristics.
(2) Safety Data
1.Pharmacokinetic Experiments
The research team conducted a series of experiments on half-life, in vivo distribution, and oral bioavailability. Comparative analysis was also done with publicly available data on drugs such as ARV-471 and ERD-3111. The results are as follows:
(1) CZ-501 exhibits moderate drug clearance rate and a relatively long half-life, indicating its ability to remain stable in the body and ensure the duration of drug action.
(2) CZ-501 has a large apparent volume of distribution, suggesting extensive distribution within the body. This indicates good lipophilicity, making it easily absorbed by cancer cells by penetrating cell membranes.
(3) The moderate AUC (area under the curve) value reflects the stable presence of CZ-501 in the body, consistent with the half-life data. At the same time, it minimizes the potential issues of drug toxicity associated with prolonged exposure.
(4) The low oral bioavailability (F < 10%) suggests that CZ-501 is more suitable for intravenous administration (consistent with the mainstream administration route for PROTACs).
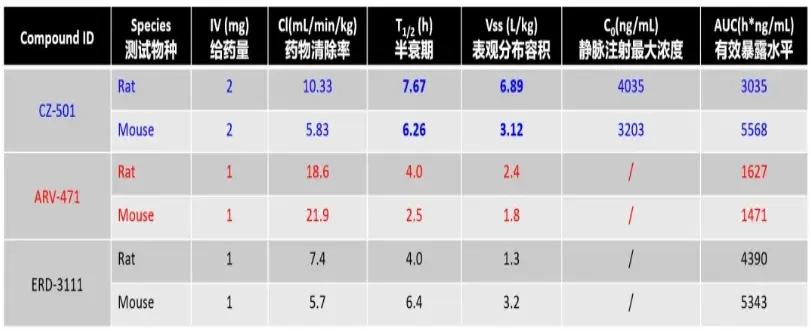
Data source: J. Med. Chem. 2023, 66, 17, 12559–12585.
2.Toxicology Experiments
Data from liver microsome metabolism experiments indicate that CZ-501 undergoes moderate metabolism in the liver microsomes. This ensures a certain level of stability for its pharmacological efficacy while also avoiding potential toxicity from drug accumulation.
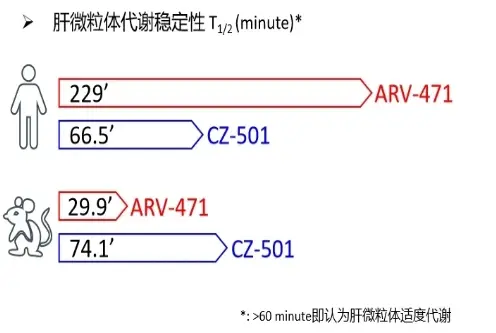
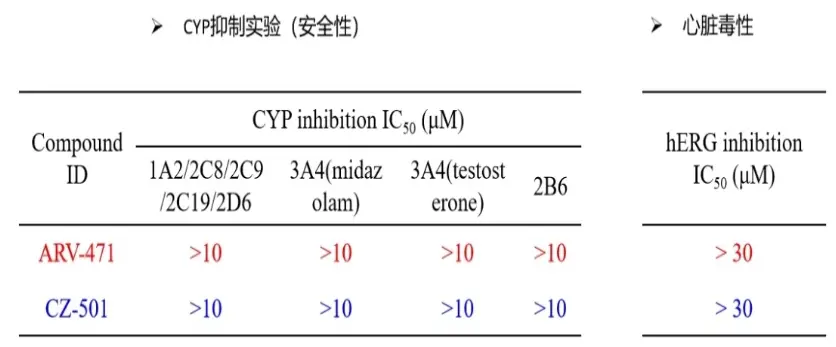
Toxicology experiments have shown that CZ-501 does not exhibit inhibitory effects on P450 enzymes and hERG enzymes, indicating excellent drug safety.
The company is currently expanding sample size and experimental scope to expedite the progress of in vitro and in vivo animal safety and efficacy experiments. It is expected to enter the IND application phase in the first half of 2025.
Regarding PROTAC:
Proteolysis targeting chimera (PROTAC) technology is an emerging targeted protein degradation technique. This breakthrough approach utilizes the cell's own degradation system to eliminate disease-causing proteins, providing a promising research strategy in drug design.
The concept of PROTAC was initially proposed by Sakamoto and Crews in 2001 when they designed the first peptide-based PROTAC molecule called PROTAC-1, which targeted the degradation of methionine aminopeptidase 2 (METAP2). PROTAC selectively induces protein degradation by utilizing the ubiquitin-proteasome system. Structurally, it consists of three components: a ligand that binds to the target protein, a linker, and a ligand that binds to E3 ligase. Over the past 20 years, an increasing number of PROTAC molecules targeting various proteins have been reported, particularly in the field of malignancies, and some have been developed as candidates for clinical trials.
Novel PROTAC degraders possess anticancer potential, favorable drug properties, and can effectively address targeting challenges and drug resistance issues that traditional therapies struggle with. As of the end of 2022, there were at least 20 PROTAC projects in clinical trials worldwide. Clinical-stage drugs such as Bavdegalutamide, ARV-471, and NX-2127 have demonstrated the activity and future potential of PROTAC in cancer treatment. PROTAC technology can target a wide range of proteins, offering broad prospects for clinical applications. With continuous maturity, development, and application of this technology, more patients are expected to benefit from it.
MoMed Biotech R&D Platform
MoMed Biotech MechGen multidimensional intelligent R&D technology platform adopts cutting-edge Nobel Prize-winning technology and deeply studies the target protein mechanism, drug molecule virtual screening, and novel artificial intelligence drug design optimization methods, among other drug discovery process technologies. It has the technical strength to quickly obtain high-quality lead compounds. CZ-501 is one of the company's most important achievements based on this platform.
The platform includes a high-performance target protein mechanism research platform, a high-throughput drug molecule virtual screening platform, a high-intelligence artificial intelligence drug design platform, a high-level drug molecule design optimization platform, a large-scale drug molecule fragment database platform, and a high-efficiency multi-pipeline drug R&D platform, covering the entire drug design process and providing comprehensive one-stop technical services.
Project Background
Breast cancer is the most common malignant tumor in the epithelial tissue of the breast, and its mortality rate ranks first among female malignant tumors. Endocrine therapy to suppress estrogen is the preferred strategy for hormone receptor-positive breast cancer treatment. However, drug resistance issues with aromatase inhibitors and selective estrogen receptor modulators are one of the main reasons for clinical treatment failure of breast cancer, which is currently a challenging problem that needs to be solved clinically.
Estrogen receptor (ER) is a steroid hormone receptor and also an estrogen-regulated transcription factor that plays a critical role in the occurrence and proliferation of breast cancer. According to statistics, nearly 80% of breast cancer is related to abnormal ER function, and ERα is an important target for endocrine therapy in breast cancer. The use of PROteolysis TAgeting Chimeras (PROTACs) that degrade ERα protein via the proteasome pathway has become a major treatment option for postmenopausal endocrine-resistant breast cancer or advanced metastatic ERα-positive breast cancer patients.
In October 2023, MoMed Biotech's R&D data preliminarily confirmed that the team's designed PROTAC protein degrader targeting ER can significantly reduce the ERα protein level in human breast cancer MCF-7 cells, and its activity and other data have reached the best level in the field.
Hot News
- 2024-10-31
- 2024-11-22
- 2025-01-16
-
2025-01-17
MoMed Biotech's Moments on CCTV! Catch the Wonderful Highlights in Advance!




