Breakthrough | Momed Biotech Discovers Novel Mechanism-Based Low-Toxicity Molecule for Parkinson’s Disease
Recently, Momed Biotech has utilized its MechGen drug discovery platform to precisely identify novel neuroprotective pathway targets from massive datasets and lock in a lead drug molecule for Parkinson’s disease treatment. The molecule demonstrated promising results in preclinical studies, showing potential to rapidly advance into Phase II clinical trials for neurodegenerative indications—highlighting the formidable advantages of AI-aided drug discovery.
Background IntroductionBckground Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disorder worldwide, is characterized by the progressive degeneration of dopaminergic neurons, leading to classic symptoms such as bradykinesia and muscle rigidity. While traditional medications like levodopa can relieve symptoms in the short term, long-term use is associated with diminishing efficacy and motor complications, highlighting the unmet need for improved therapeutic strategies.
Research Breakthroughs
Using its proprietary MechGen drug discovery platform—a novel mechanism-target discovery engine—Momed Biotech recently leveraged big data mining models to pinpoint a novel neuroprotective pathway target from massive datasets. The company then applied its virtual screening and drug repurposing platforms to identify a Phase II oncology candidate molecule targeting this pathway, testing its neuroprotective effects in Parkinson’s disease (Figure 2).
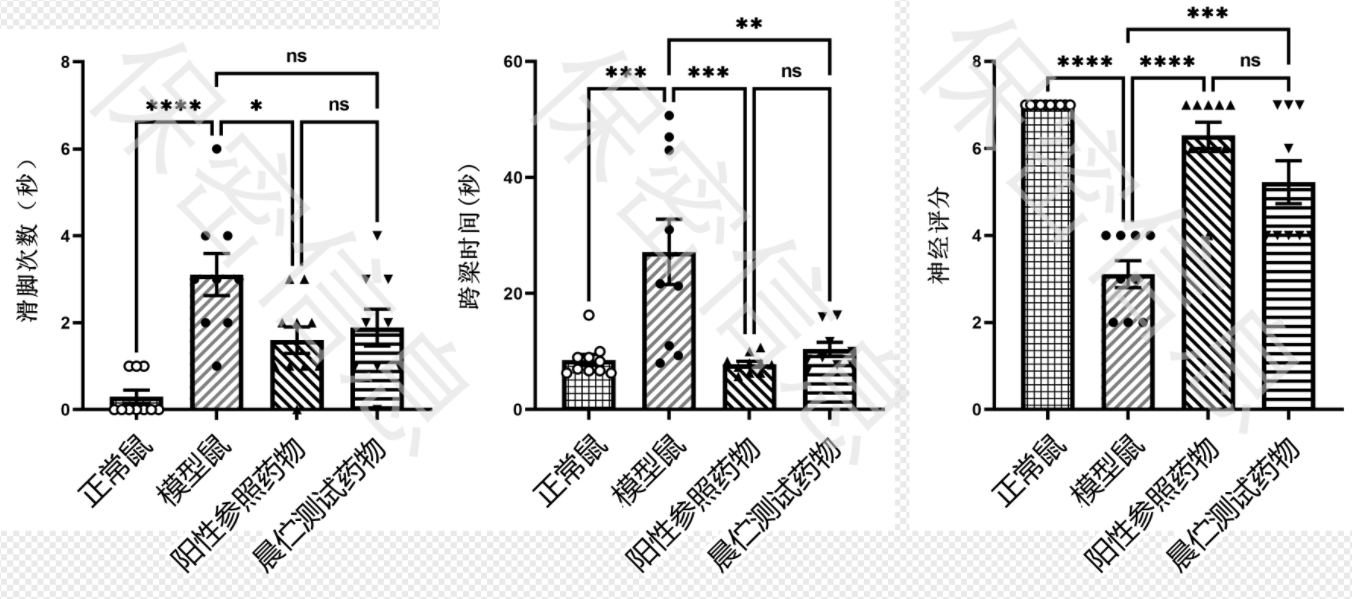
In safety evaluations, no significant changes were observed in physiological indices such as body weight and food intake in treated mice, with no drug-related adverse events—confirming the molecule’s favorable tolerability and safety profile. Pharmacokinetic studies showed excellent blood-brain barrier permeability, enabling efficient delivery to the central nervous system. Behavioral tests demonstrated significant improvement in motor function in treated mice, comparable to the positive control drug selegiline, further validating its therapeutic potential for Parkinson’s disease. These promising results provide strong support for advancing the candidate molecule, positioning it as a promising new treatment option.
The candidate molecule developed by Momed Biotech offers three significant advantages over traditional Parkinson’s disease therapies:(1) it acts through a novel neuroprotective mechanism to protect neurons, opening a groundbreaking therapeutic route for PD; (2)compared with existing drugs, it demonstrates exceptionally low toxicity, indicating superior safety and tolerability profiles; (3)it can be used as a standalone therapy or in combination with other drugs, enabling diverse and personalized treatment strategies.
Given its unique neuroprotective mechanism, Momed Biotech previously tested the drug in Alzheimer’s disease (AD) and achieved promising results, with its strong performance in Parkinson’s-related experiments further validating its broad potential for treating neurodegenerative disorders. This discovery not only highlights the company’s technical edge in drug repurposing but also paves the way for new treatment possibilities across multiple neurological diseases, with Momed Biotech delivering more targeted and effective treatment options to patients worldwide through this innovative development model. Clinical trial application data collection for both PD and AD indications is currently underway, and since the molecule has already advanced to Phase II trials in oncology, it is poised to rapidly enter Phase II testing for neurodegenerative indications.
Momed’s AI-Driven Advantages
References:
1.Wikipedia-Parkinson's disease
2.Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.
Hot News
- 2024-10-31
- 2024-11-22
- 2025-01-16
-
2025-01-17
MoMed Biotech's Moments on CCTV! Catch the Wonderful Highlights in Advance!




