JACS | The Bai Chen team and collaborators reveal the activation mechanism and important mutation sites of the ion channel TMEM16A through computational simulations.
On February 6, 2024, the Bai Chen team from MoMed Biotech, along with collaborators, published a research paper titled "Predicting Mutational Effects on Ca2+-Activated Chloride Conduction of TMEM16A Based on a Simulation Study" in the JACS journal online.
Dysfunction and defects in ion channels are associated with many human diseases, particularly loss-of-function mutations in ion channels such as those in cystic fibrosis transmembrane conductance regulator.
Therefore, understanding ion channels is crucial for both medical and basic research, including predicting the effects of key residue mutations in ion channels, describing the ability of ion channels to function and their mechanical effects, and so on.
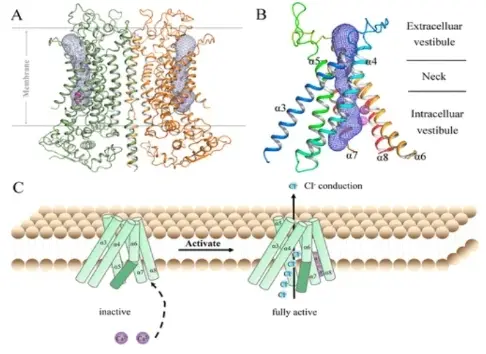
Figure 1. Structure of TMEM16A and the Ca2+-activated chloride conduction pathway.
In this study, the authors proposed a method to investigate protein mechanisms and predict mutation effects based on dynamic information obtained by studying the working mechanism of the target protein, including reaction barriers and transition state positions.
Specifically, using the Ca2+-activated chloride ion channel TMEM16A as an example, the authors used a computational biology model to couple multiple important events in protein function and predict the mutation effects of key residues by calculating free energy.
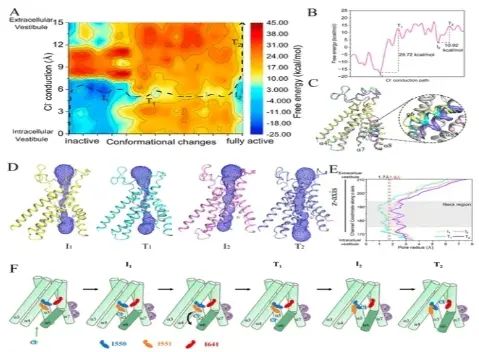
Figure 2. Coupling between conformational changes in TMEM16A and chloride ion conduction.
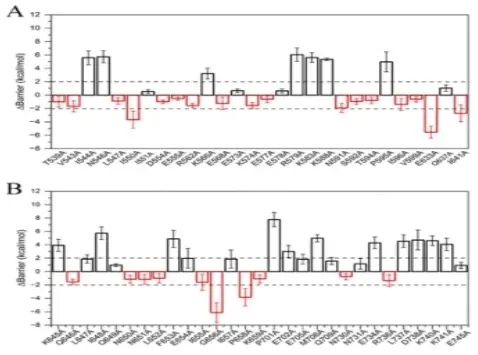
Figure 3. Mutation effects analysis of residues in two vestibules, Ca2+ binding sites, and the pore lining neck region of TMEM6A.
The research and development team validated the computational predictions with electrophysiological experiments, and the results showed a high accuracy of 94% in predicting the direction of mutations.
Furthermore, there was a strong negative correlation (Pearson correlation coefficient of -0.80) between the computational and experimental results.
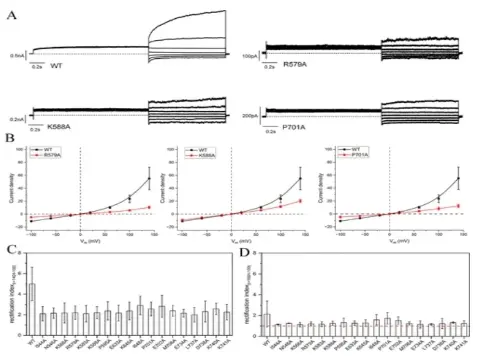
Figure 4. Electrophysiological validation experiments.
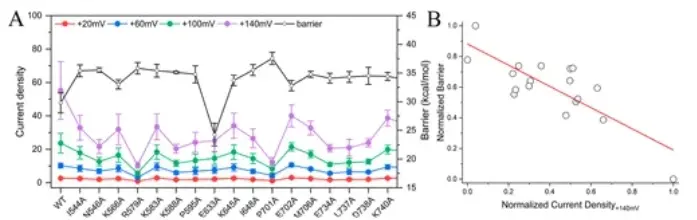
Figure 5. Comparison of current density in electrophysiological experiments with calculated energy barriers.
These findings indicate that the method proposed by the research team, which involves studying the working mechanism of the target protein to obtain dynamic information (including reaction barriers and transition state positions) for predicting mutation effects, is reliable and effectively applicable in elucidating the functional mechanism of the TMEM16A channel and determining key residues.
Such methods can be extended to a broader range of biophysical systems and applied in various aspects such as drug design.
The Chinese University of Hong Kong (Shenzhen), University of Science and Technology of China, Shenzhen University Affiliated South Hospital, University of Southern California, and MoMed Biotech were joint collaborative units for this research paper.
Link to the original research paper:
https://pubs.acs.org/doi/10.1021/jacs.3c11940
Hot News
-
2022-11-05
The Bai Chen Research Group Publishes Paper in the International Journal of Molecular Sciences
- 2022-12-20
- 2022-12-26
- 2026-02-25




