JACS | Bai Chen's team from MoMed Biotech reveals the activation mechanism of the important chloride ion channel protein glycine receptor in the nervous system through computational simulation.
In September 2024, Bai Chen's team from MoMed Biotech published a research paper titled "Exploring the Activation Process of the Glycine Receptor" online in the journal JACS. This study not only helps us deeply understand the activation mechanism of the glycine receptor (GlyR), a potential therapeutic target for alleviating chronic pain, predicts and validates important mutation sites, but also opens up a new way for developing more effective treatment regimens.
The GlyR belongs to the pentameric ligand-gated ion channel family and is an important chloride ion channel-type protein located in the central nervous system that conducts nerve signals through glycine. Abnormal function of GlyR can lead to diseases such as epilepsy, chronic pain, and excessive startle.
Studies have shown that GlyR can inhibit pain signals in the dorsal horn of the spinal cord and is expected to be a new target for treating chronic pain, addressing the shortcomings of current analgesics (such as opioids) with limited effectiveness and high addiction. However, due to the very complex activation process of GlyR, our understanding of its activation mechanism is still insufficient, which largely limits our progress in understanding its function and drug design.
Research test
In this work, the research team used computational biology models to study the effects of different numbers of agonist bindings on the activation and desensitization behaviors of GlyR and explored the reason why the lateral path is the main conduction path for chloride ions in terms of energy.
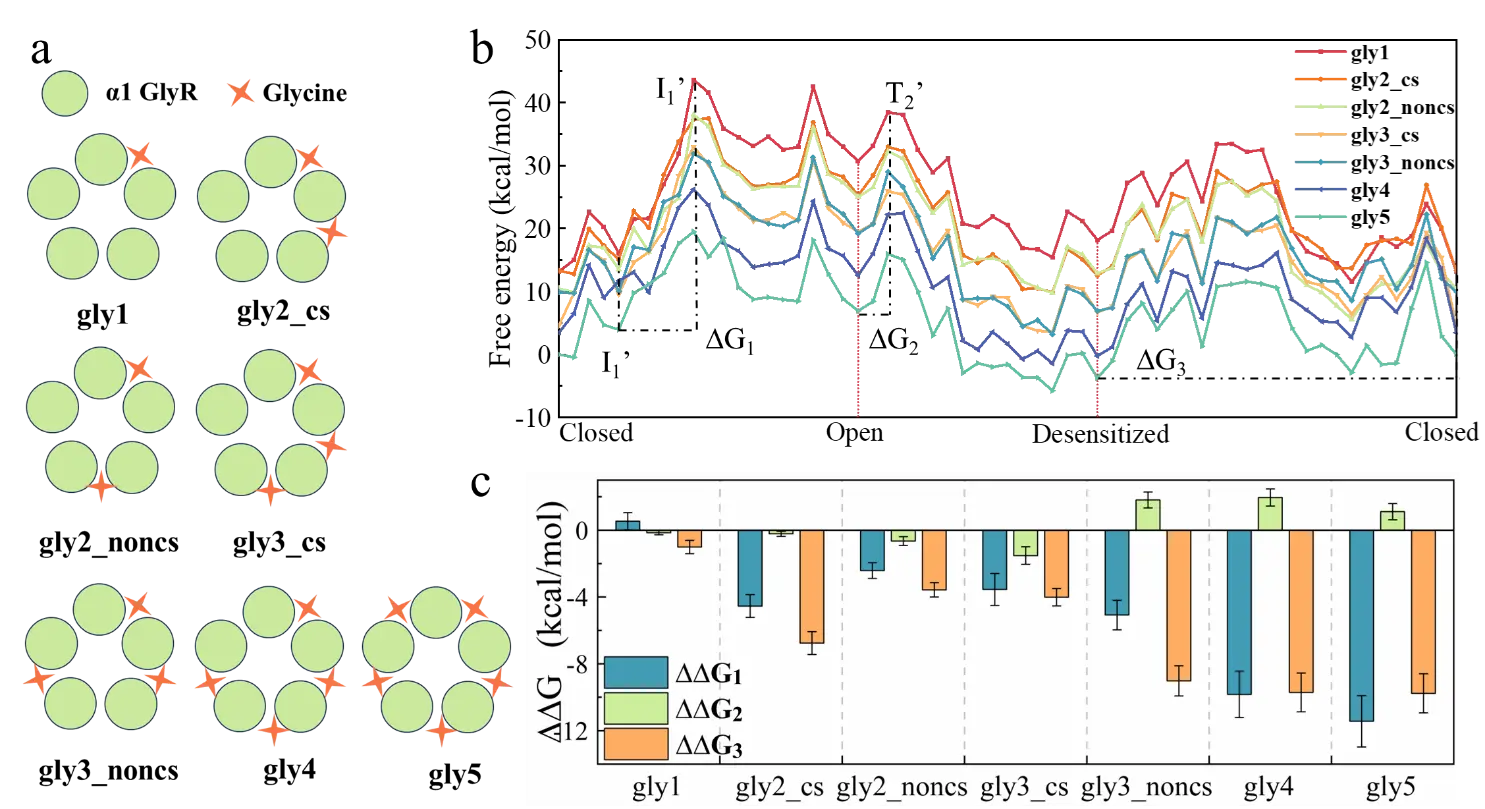
Figure 1. Effects of different glycine binding modes on the activation and desensitization behaviors of GlyR. (a) Seven glycine molecule binding modes. (b) Energy changes of protein conformational energy coupling glycine binding energy in different binding modes. (c) ∆G changes caused by different glycine binding modes. ∆G1: activation energy barrier, ∆G2: desensitization energy barrier, ∆G3: energy difference between closed state and desensitized state.
Through molecular-level simulation and energy analysis, the research team revealed that increasing the number of molecular bindings helps activate GlyR and inhibit GlyR desensitization, and explained why increasing glycine concentration makes the desensitized state the main state.
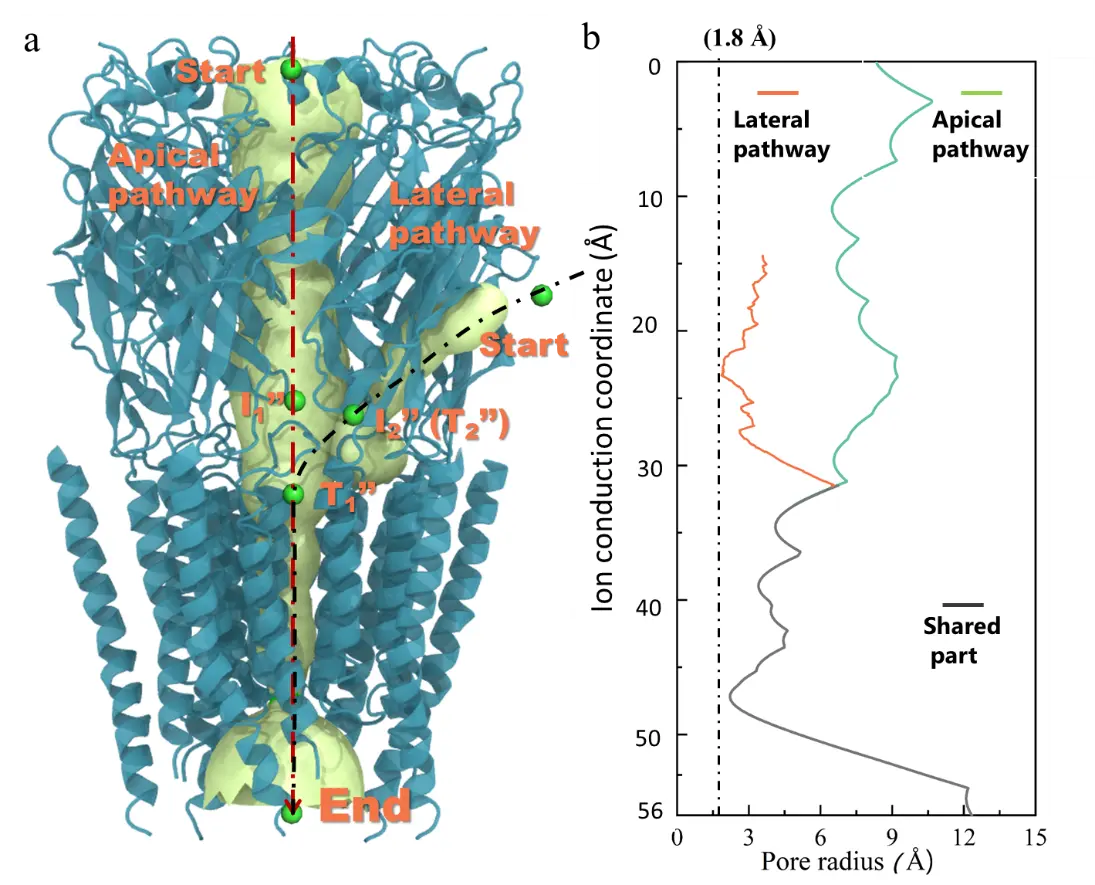
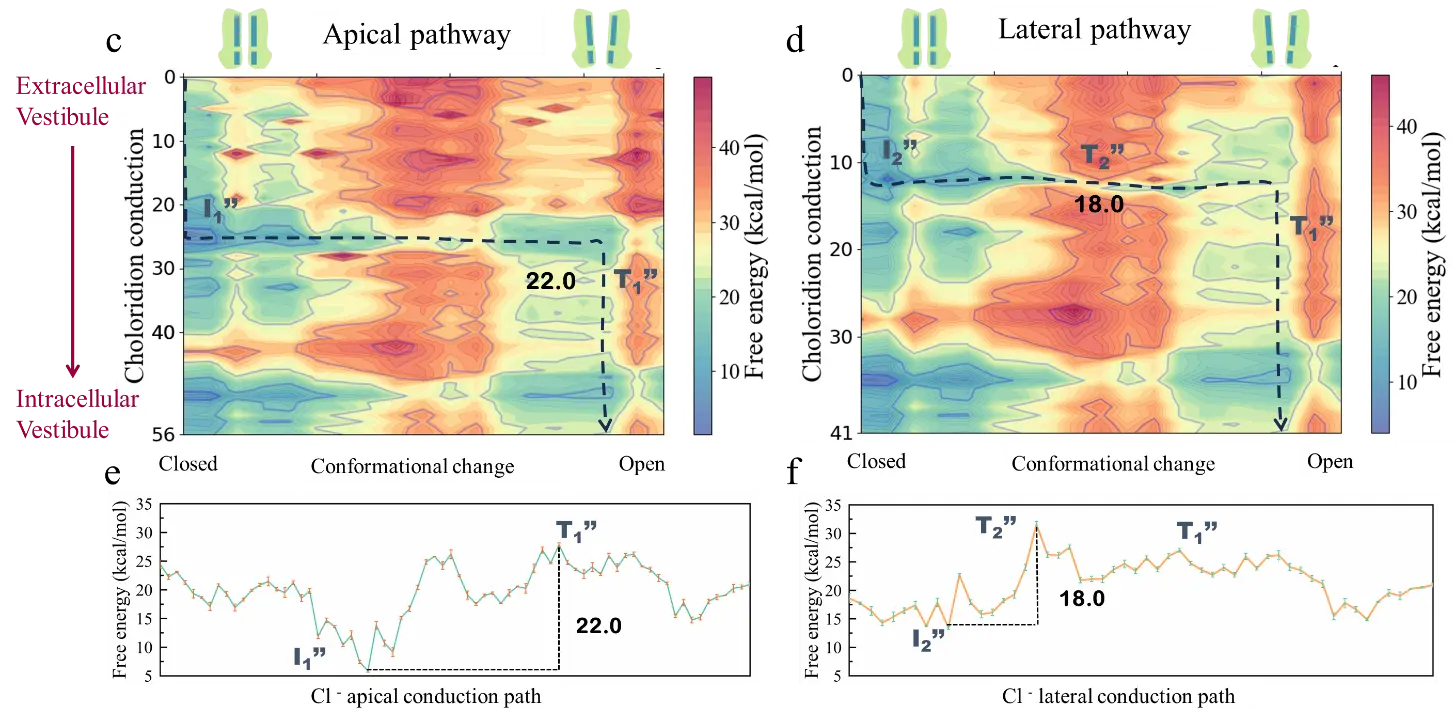
Figure 2. Energy differences in chloride ion conduction through the top and lateral paths. (a) Distribution of top and lateral paths on GlyR. (b) Changes in channel pore size of top and lateral paths. (c) and (d) Free energy landscapes of chloride ion conduction through top and lateral paths. (e) and (f) Free energy curves of the minimum energy paths corresponding to figures (c) and (d).
To explore the energy difference in chloride ion conduction through the top and lateral paths, the research team constructed free energy landscapes of ion conduction through the lateral and top paths and found that the energy barrier for chloride ion conduction is lower in the lateral path. Subsequent electrostatic potential energy surface analysis shows that the electrostatic environment plays an important role.
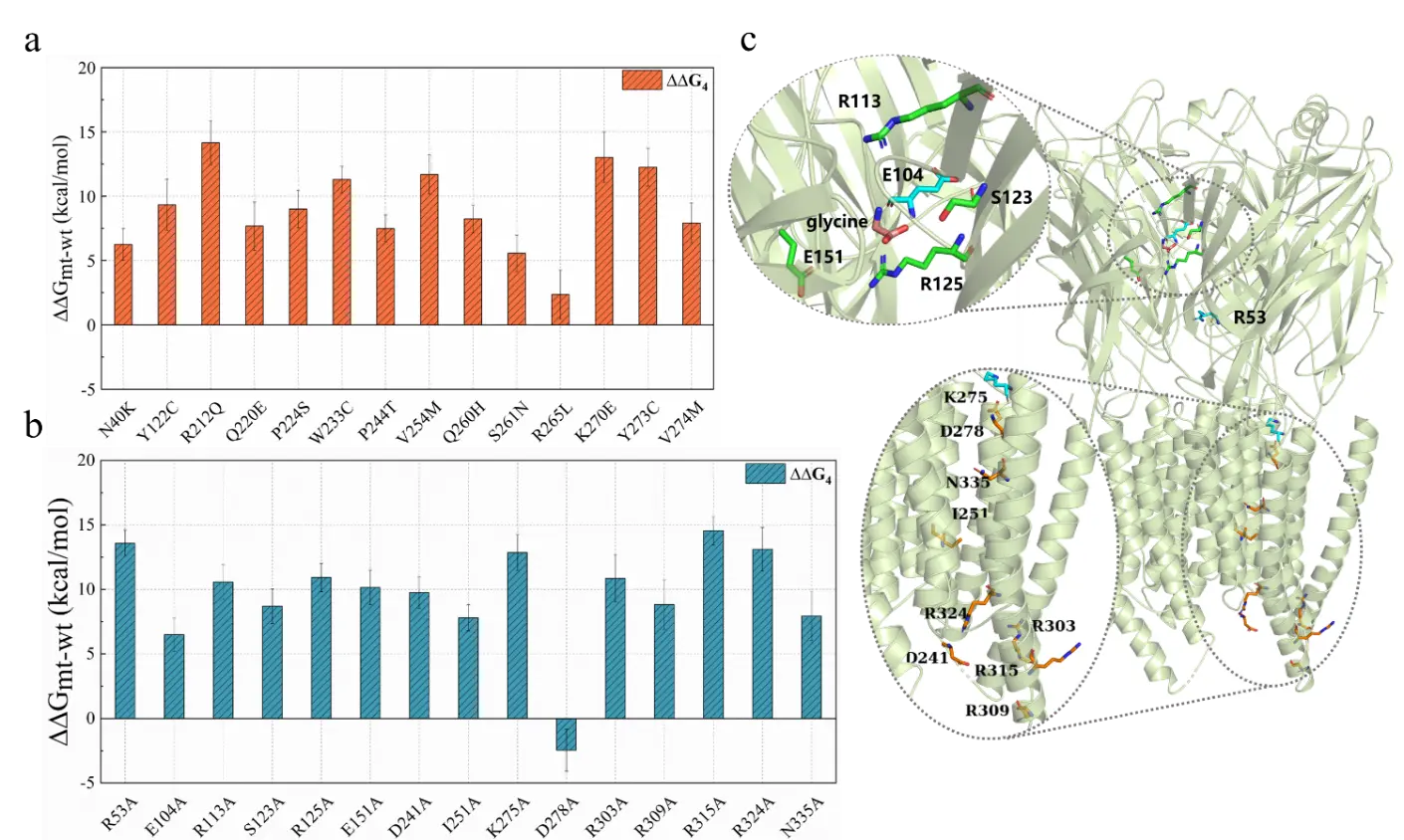
Figure 3. Mutation effect analysis of residues on GlyR located at glycine binding sites, chloride ion conduction paths, and key loop regions.
Subsequently, the research team also conducted mutation effect analysis on residues on GlyR located at glycine binding sites, chloride ion conduction paths, and key loop regions. This helps identify key residues affecting the GlyR activation process and provides a direction for future mutation effect studies.
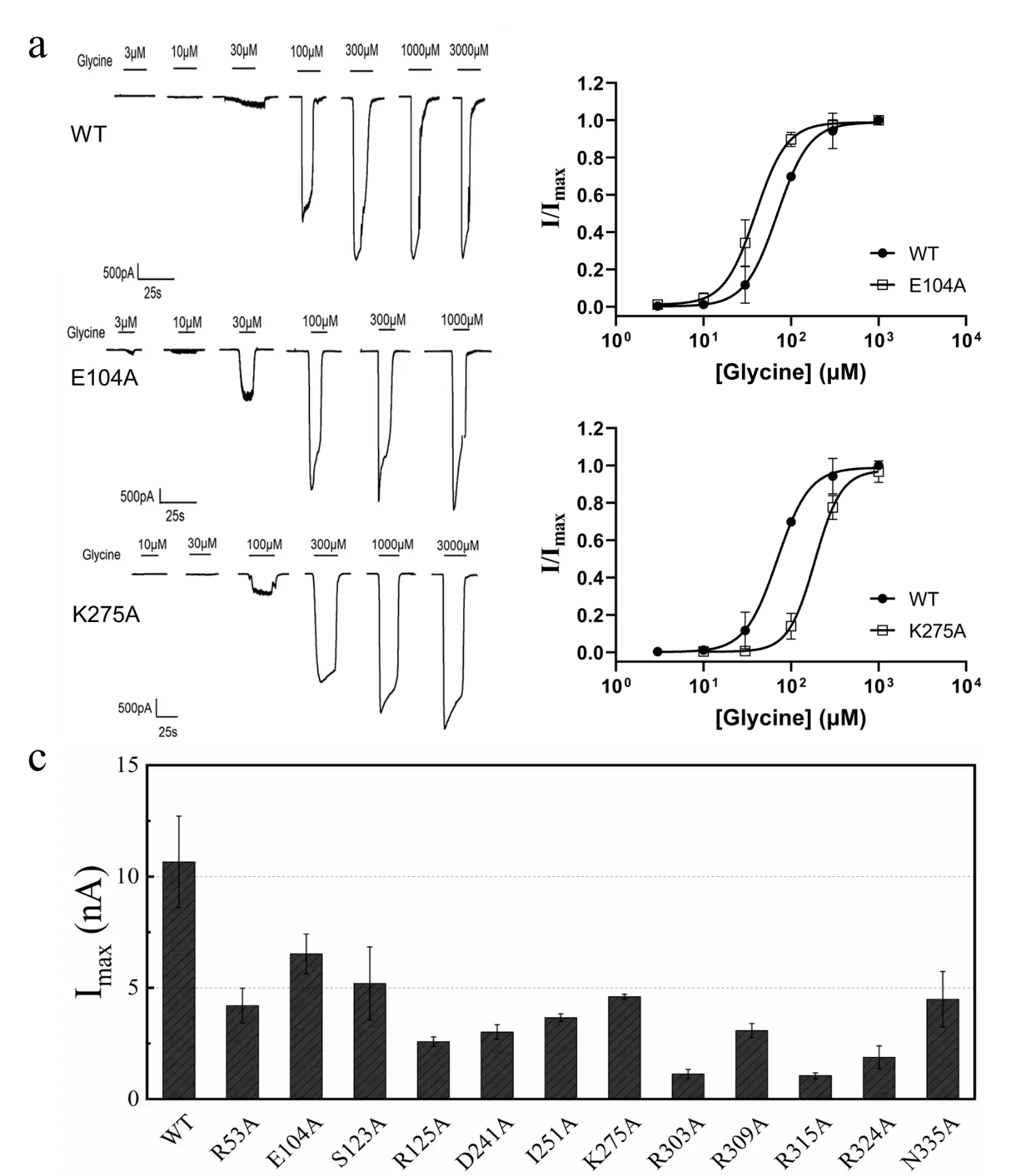
Figure 4. Electrophysiological verification experiment.
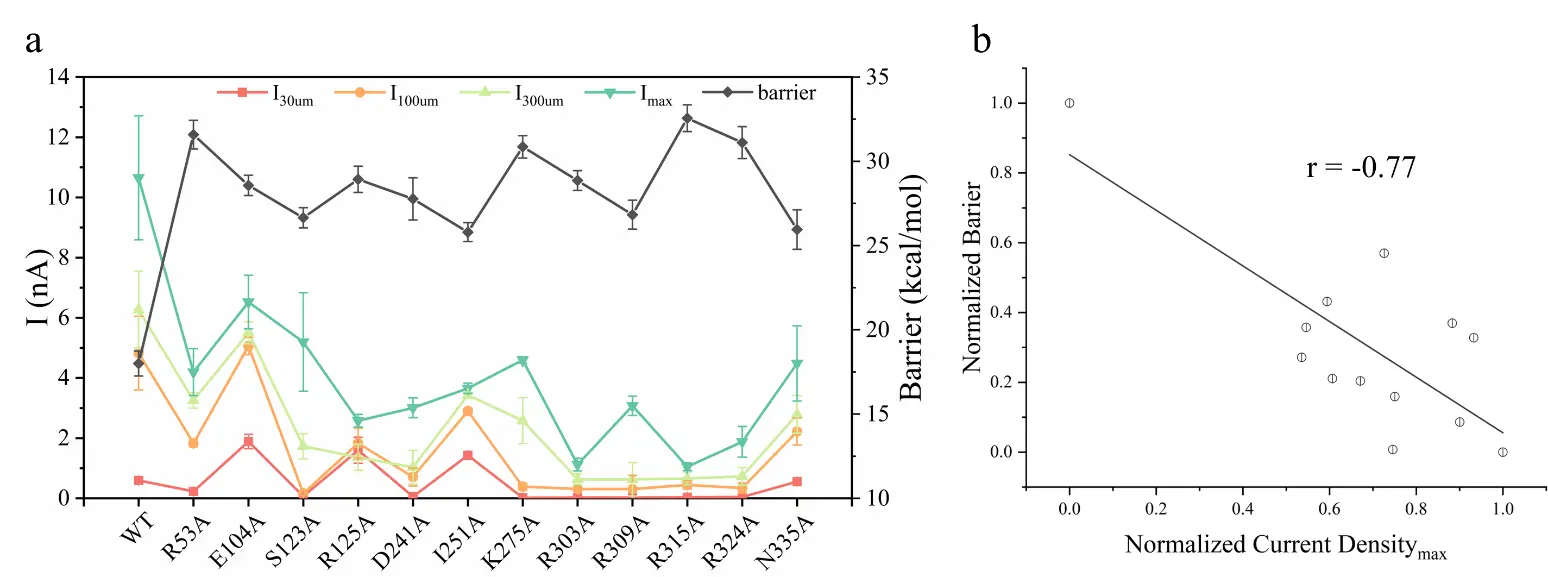
Figure 5. Comparison of current density in electrophysiological experiments and energy barriers of calculation results.
The research and development team combined electrophysiological experiments to verify the computational predictions. The study shows that for all mutants that can be activated by glycine, the prediction of the mutation direction is completely accurate.
Finally, the computational results and experimental results also show a good negative correlation (Pearson correlation coefficient is -0.77).
In conclusion, this study not only theoretically expands our understanding of the activation mechanism of GlyR but also provides valuable information and inspiration for the design and development of GlyR drugs in practical applications.
With a deeper understanding of the activation mechanism of GlyR, we have reason to believe that more innovative therapies for central nervous system diseases will emerge in the future, bringing new hope to patients.
The Chinese University of Hong Kong, Shenzhen, Zhejiang University, MoMed Biotech, and the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences are the co-authoring institutions of this paper.
Interested readers can read the original research paper:
https://pubs.acs.org/doi/10.1021/jacs.4c08489#
Hot News
-
2022-11-05
The Bai Chen Research Group Publishes Paper in the International Journal of Molecular Sciences
- 2022-12-20
- 2022-12-26
- 2026-02-25




